MINDCOVID focuses its research on providing a comprehensive assessment of the impact of the current Covid-19 pandemic on the mental health of the Spanish population.
Study design and population
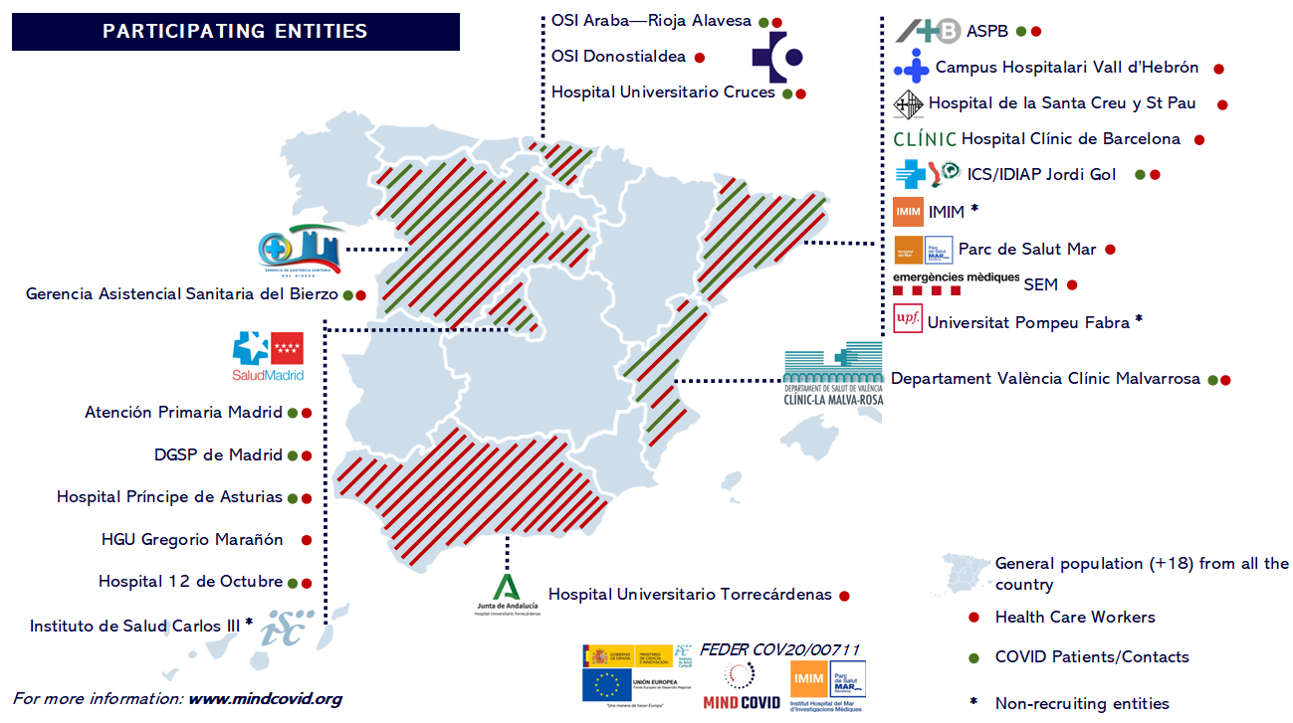
Participating healthcare professionals came from 18 healthcare institutions in 6 autonomous communities in Spain (Andalusia, Basque Country, Castile and Leon, Catalonia, Community of Madrid, and Valencian Community). The institutions were selected to reflect the geographic and sociodemographic variability in Spain; most of the participating centers were from regions with a large number of COVID-19 cases. One baseline and three follow-up assessments were conducted through participants' institutional e-mail using the Qualtrics(R) platform.
Baseline assessment
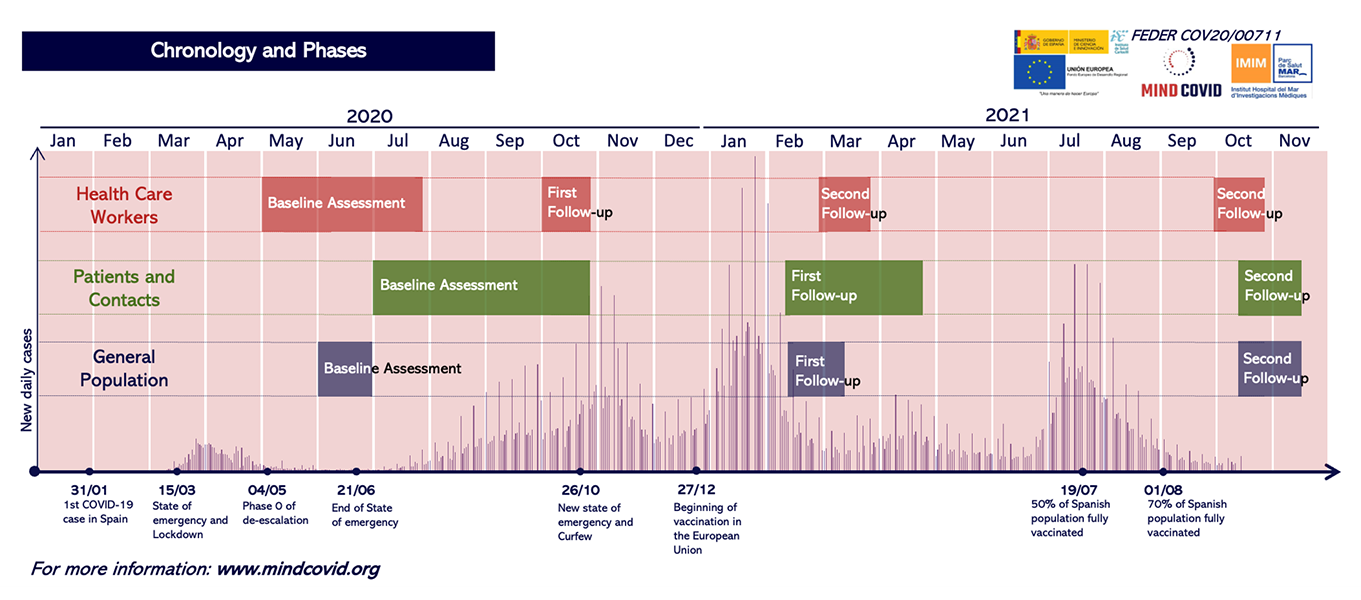
The baseline assessment consisted of anonymous self-reported web-based surveys administered shortly after the first outbreak of COVID-19 in Spain. Data collection was initiated at a time when the number of new cases in Spain stabilized, but healthcare institutions-especially hospitals-were under very high pressure of demand (May 5th to September 7th, 2020).
All workers employed in each participating health institution were invited to participate using the centers' institutional e-mail distribution lists (i.e., the target sample was the census of workers). The survey was not further advertised and no incentives for participation were offered.
The invitation email included an introductory text and an anonymous link to access the web-based survey platform (qualtrics.com). Informed consent was obtained from all participants on the first page of the survey. Up to two reminder emails were sent within 2 to 4 weeks after the initial invitation. At the end of the survey, all participants received a detailed list of local mental health resources, including resources for nearby urgent care for respondents reporting a suicide attempt in the previous 30 days.
Follow-up assessments
A first follow-up was conducted approximately 2-4 weeks after answering the baseline questionnaire. Data collection was conducted between June 2020 and October 2020. Reminders were sent at 5 and 10 days. The invitation maintained the format used in the baseline questionnaire via website (qualtrics.com).
A second follow-up was performed approximately 4 months after answering the baseline questionnaire. Data collection took place between October 2020 and November 2020. Between 3 to 4 reminders were sent within 2 weeks. The invitation maintained the format used in the baseline questionnaire via the website (qualtrics.com).
A third follow-up was performed approximately 9 months after answering the baseline questionnaire. Data collection took place between March 2021 and April 2021. Four reminders were sent within 2 weeks.The invitation maintained the format used in the baseline questionnaire via website (qualtrics.com).
Baseline assessment participation
A total of 9,138 healthcare workers participated in the baseline survey. The response rate is difficult to estimate, since the survey viewing rate (i.e., the proportion of health care workers who opened the invitation e-mail) is unknown, except in one hospital facility (which was 26.4%). When the denominator used to calculate the response rate is the total number of health care workers on the e-mail distribution list or the total number of health care workers employed as provided by hospital representatives, the survey response achieved, adjusted for sample size, was 12.5%. The survey participation rate (i.e., those who agreed to informed consent to participate divided by the total number of people who accessed the first page of the survey with the study information) was 89.0%, and the survey completion rate (i.e., those who completed the survey among those who agreed to participate) was 80.8%.
Participation in follow-up evaluations
At the FIRST FOLLOW-UP (2-4 WEEKS), 7,528 health care workers were invited, i.e., 100% of those who answered the baseline questionnaire and gave their e-mail address. A total of 4,959 responses were received, so participation was 66%.
At the SECOND FOLLOW-UP (4 MONTHS), 7,393 health care workers were invited to participate. A total of 4,786 responses were received, obtaining a participation rate of 65%.
Finally, at the THIRD FOLLOW-UP (9 MONTHS), 7,349 health workers were invited to participate. A total of 4,732 responses were received, so participation was 64%.
Study design and population
A sample of COVID-19 cases and contacts was selected from 10 healthcare institutions from 5 autonomous communities in Spain (Basque Country, Castile and Leon, Catalonia, Community of Madrid, and Valencian Community). The institutions were selected to reflect the geographic and sociodemographic variability in Spain; most of the participating centers were from regions with a large number of COVID-19 cases. One baseline and two follow-up assessments were conducted through participants' institutional e-mail, using the Qualtrics(R) platform, and through telephone interviews, using the GESOP platform.
Each of the participating centers conducted its own recruitment of cases and/or close contacts from COVID-19. For this purpose, a sampling frame was established consisting of the total number of cases or contacts in the center in a given period of time, and a quota of patients to be recruited was established, stratified by age and date of diagnosis or close contact.
The sampling frame was obtained from information from the different local and regional epidemiological services and from the different health services (hospitals and primary care centers) responsible for the identification and management of COVID-19 patients and their close contacts.
Recruitment was carried out both in person and by telephone calls from the participating health centers. For those recruited in person, a visit to the health center or at discharge was used to inform them about the study and invite them to participate, while those who were invited by telephone were recruited retrospectively, using the available contact information.
Baseline assessment
The baseline assessment consisted of anonymous self-reported surveys via the website (qualtrics.com) or by computer-assisted telephone interviews (CATI) carried out by a specialized company or by persons hired at the centers. Data collection began in July 2020 and ended in March 2021.
Informed consent was again obtained from the participants as the first question of the survey. For those invited via web, 4 reminders were sent within 2 weeks. For those interviewed by telephone, contact was attempted up to 3 times. At the end of the survey, all participants received a detailed list of local mental health resources, including resources for nearby urgent care for respondents reporting a suicide attempt in the previous 30 days.
Follow-up assessments
In those patients who answered the baseline questionnaire via the website (qualtrics.com), a first follow-up was conducted approximately 2-4 weeks after answering the baseline questionnaire. Data collection was performed between July 2020 and April 2021 using the same web-based format (qualtrics.com). Reminders were sent at 5 and 10 days after the baseline questionnaire.
A second follow-up was performed approximately 7 months after answering the baseline questionnaire. Data collection took place between February 2021 and July 2021. Those who were invited via the website were sent 3 to 4 reminders in 2 weeks, while those who answered via telephone were contacted up to 3 times.
Baseline assessment participation
A total of 4,185 COVID-19 patients were recruited and 1,009 contacts agreed to participate in the study. Of these, 2,660 responses were finally obtained, of which 1,600 were via the Qualtrics platform and 1,060 were by telephone. Therefore, participation was close to 51%
Participation in the follow-up evaluations
A total of 1,465 COVID-19 patients and contacts were invited to the first follow-up questionnaire (2-4 weeks), that is, 100% of the people who answered the baseline questionnaire via the Qualtrics platform and accepted the informed consent. A total of 1,091 responses were received, so participation was 74%.
Study design and population
This corresponds to a representative sample of the general adult Spanish population. To select the sample, a double frame telephone sampling was carried out, including a sample of cell phone numbers and another of landlines. A list of cell phone numbers was generated by an automated system, and landline numbers were selected through an internal database developed and maintained by the company, with the aim of ensuring that all Spanish autonomous communities were correctly represented. Up to seven calls were made at different times of the day and several times a week to each selected number. A total of 138,656 numbers were sampled, aiming for representativeness of the sample in terms of age, sex and autonomous community. Verbal informed consent was obtained for data collection from the participants.
The inclusion criteria were: (1) being over 18 years of age and not institutionalized, (2) being a resident in Spain at the start of the study, and (3) having a landline or cell phone. In turn, the exclusion criteria were: (1) having a language barrier to being able to understand the language of the survey and (2) not giving explicit consent to participate.
A baseline and a follow-up assessment was carried out via computer-assisted telephone interviews with trained interviewers by a company specializing in research services, IPSOS.
Baseline assessment
The baseline assessment was conducted via computer-assisted telephone interviews between June 1 and June 30, 2020.
Follow-up assessments
A follow-up was conducted approximately 9 months after answering the baseline questionnaire, between February 18 and March 12, 2021.
To incentivize participation in both surveys, at the beginning of each survey, participation in a raffle for 8 state-of-the-art laptops was offered upon completion of both surveys.
Baseline assessment participation
Of the 138,656 numbers sampled, 45,002 were ineligible (business, non-existent number, language barrier, etc.) and 72,428 were of unknown eligibility as they did not respond to any of the calls. The cooperation rate (i.e., the proportion of individuals interviewed among all eligible callers) was 16.5%, with 75% being mobile numbers and 25% being landline numbers, with 3,500 participants in total completing the survey.
Participation in follow-up evaluations
In the follow-up evaluation (9 months), an attempt was made to contact the 3,500 people who answered the baseline questionnaire. A total of 2,000 people participated, so the participation rate was 57%.
The baseline questionnaire is divided into thematic blocks and is made up of Spanish versions of widely accepted and validated instruments in psychological and psychiatric research and practice. Thematic blocks assess relevant mental health outcomes, sociodemographic variables, COVID-19 infection status, and a range of relevant risk and protective factors for adverse mental health related to the COVID-19 pandemic. The following measures were used to screen for adverse mental health outcomes and, hence, to determine the current presence of a probable mental disorder::
The following items, among others, have been included in the follow-up questionnaires:
The study complied with the principles established by national and international regulations, including the Declaration of Helsinki and the Code of Ethics. The study protocol was approved by the Medical Research Committee (CEIm) Parc de Salut Mar (2020/9203 / I) and by the corresponding CEIm of all participating centers. The study is registered in ClinicalTrials.gov.